How to Get FDA Approval with One Clinical Trial and Confirmatory Evidence
“Regulatory flexibility” allows some drugs to be approved based on a single adequate and well-controlled clinical trial with confirmatory evidence. This regulatory pathway can be highly valuable when developing drugs for rare or serious diseases. But what does that mean and how can it be incorporated into a drug development program?
This blog post delves into the concept of confirmatory evidence, drawing from the recent draft FDA guidance. It provides some examples of the types of data that can be used to support a pivotal clinical trial and shares some tips on how to engage with the FDA to maximize the chances of regulatory and commercial success.
What is confirmatory evidence?
According to FDA guidance1,2:
Confirmatory evidence is any data or information that can substantiate the results of a single pivotal clinical trial and increase the certainty around the drug’s safety and effectiveness.
Confirmatory evidence can come from various sources:
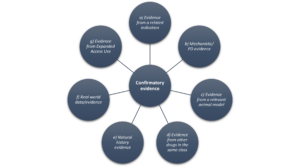
- Clinical evidence from a related indication
- This involves the utilization of data derived from a separate clinical trial of the same drug in a different indication. Instances where trial data from related indications might serve as confirmatory evidence include studies focusing on a different stage of the same disease or closely related conditions.
- Mechanistic or pharmacodynamic evidence:
- A comprehensive understanding of disease pathophysiology and a clear grasp of a drug’s mechanism of action (MoA) may allow mechanistic or pharmacodynamic evidence to be considered confirmatory, in addition to robust evidence of the drug’s direct impact on disease pathophysiology. However, if the MoA influences multiple disease-relevant pathophysiological pathways, mechanistic data alone may not be adequate to provide the necessary confirmatory evidence for approval.
- Evidence from a relevant animal model(s):
- If compelling evidence exists in well-established animal model(s) of disease, with in vivo efficacy studies offering reasonable support for anticipated clinical benefits and outcomes in humans, data might be considered confirmatory. FDA guidance emphasizes that ‘only models that have proved to be translational (i.e., prior drugs with the same intended clinical effect have been shown to have this effect observed in the animal model, with similar exposure-response) are likely to be considered confirmatory evidence.’
- Evidence from other drugs in the same pharmacological class:
- FDA may consider data from other drugs in the same class with the same MoA and comparable effects on clinical outcomes. The validity of these data depends on whether similar endpoints were consistently measured, and the consistency of the impact of each drug on clinical outcomes.
- Natural history evidence:
- Data from natural history studies can provide valuable information on the expected progression and outcome of a condition without treatment. This becomes relevant in scenarios where there is uncertainty about whether the outcomes observed in the control group of a single study accurately reflect what would typically occur in the general (non-studied) population. Such data can be instrumental in determining the necessity of a placebo arm in the study and can directly impact trial costs.
- Real-world data/evidence:
- Trustworthy and pertinent data gathered from routine clinical practice or alternative sources beyond clinical trials may also be considered confirmatory. These sources might include electronic health records, medical claims, registries, or expanded access programs.
- Evidence from Expanded Access Use of an Investigational Drug:
- Expanded access to an investigational drug can be authorized for various reasons. Should patient outcomes from expanded access programs be of sufficient quantity and quality, they may be used as confirmatory evidence.
The quantity and quality of confirmatory evidence required to support a single pivotal trial vary depending on the features and results of the trial being undertaken, the type and source of data, and the clinical context of the disease and the drug. A strong scientific rationale needs to be provided for the chosen approach and descriptions of the planned trial and confirmatory evidence need to be outlined in the application.
The guidance also suggests that confirmatory evidence can be aggregated from various sources and that sponsors should discuss their proposed approach with FDA early in development. The Agency warns against cherry-picking favorable confirmatory evidence and requires applications to include a description and analysis of all data or information relevant to an evaluation of the safety and effectiveness of the drug product. The draft guidance does not specify the amount or weight of confirmatory evidence required to approve a therapy: this is determined case by case, in the context of the application as a whole.
How to engage with FDA?
FDA encourages early and frequent discussions of plans to use one clinical trial and confirmatory evidence to establish substantial evidence of effectiveness. This should ideally start at a pre-IND meeting and no later than when feedback is sought on pivotal trial design. Frequent meetings with the FDA are encouraged throughout product development, especially if changes are made to trial design or the components of confirmatory evidence.
Engaging with FDA early can provide valuable input and guidance on whether an approach is appropriate and feasible, and what factors and considerations the Agency will take into account when evaluating an application. Ultimately, whether one clinical trial and confirmatory evidence are sufficient to demonstrate substantial evidence of effectiveness will depend on the results generated by the development program, making an expertly thought-through clinical development plan essential.
Adnovate’s team of highly experienced experts is experienced in creating objective science-led assessments of available preclinical and clinical data in order to broker and maximize relevant interactions with the FDA.
Conclusion
Using one clinical trial and confirmatory evidence to demonstrate substantial evidence of effectiveness is a valuable and flexible pathway for drug approval, especially for rare or serious conditions. However, it requires careful planning, execution, and regular communication with FDA. Understanding the concept and types of confirmatory evidence and following FDA recommendations and guidance can significantly increase the chances of success.
At Adnovate, we can help you to design and support clinical development programs to meet strategic objectives and formulate regulatory strategies that can optimize the chances of successful negotiations with regulatory agencies and commercial success.
Please get in touch for more information: Contact Adnovate .
- FDA issues draft guidance regarding confirmatory evidence of clinical trials. 09/18/2023. U.S. Food & Drug Administration. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-issues-draft-guidance-regarding-confirmatory-evidence-clinical-trials
- Demonstrating Substantial Evidence of Effectiveness With One Adequate and Well-Controlled Clinical Investigation and Confirmatory Evidence Guidance for Industry: Draft Guidance. 44625092dft.docx 09/11/23. Available at: Demonstrating Substantial Evidence of Effectiveness With One Adequate and Well-Controlled Clinical Investigation and Confirmatory Evidence (fda.gov)
;)